Mechanism of action of Aspirin
Aspirin is an antiplatelet drug. It stops the production of Thromboxane A2 in the platelet. This thrombixaneA2 is responsible for the vasoconstriction and coagulation process. Aspirin binds with the COX enzyme. This enzyme is responsible for forming prostaglandin G2 and then prostaglandin H2 from arachidonic acid. This prostaglandin H2 is further converted into thromboxane A2 with the help of the thromboxane A2 synthetase enzyme. Aspirin non-selectively binds with the COX enzyme and makes a permanent modification to the active site of the COX enzyme. So this enzyme does not carry any catalyzing reaction and thromboxane A2 is not formed. Aspirin works on the COX enzyme. COX enzyme is responsible for the preparation of prostaglandin which is responsible for fever, pain, inflammation, etc.
Aspirin
Aspirin is an antiplatelet, antipyretic, and analgesic drug, It is also known as Acetylsalicylic acid. This is used for the treatment of fever and pain. It has given shortly after a heart attack to decrease the risk of death. It is an odorless white, crystalline powder that is slightly bitter in taste and soluble in DMSO and ethanol. The molecular weight of aspirin is 180.1 gm/mol.
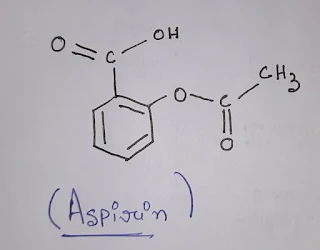
Structure:
The molecular formula of aspirin is.[C9H8O4 ].
The IUPAC name of aspirin is 2-acetyloxy benzoic acid.
Synthesis:
Uses:
- It is used to reduce fever and pain(headache, muscle ache, common cold, etc).
- Aspirin lowers the risk of a heart attack.
- It is used for blood thinning.
- It is used to reduce fever and pain(headache, muscle ache, common cold, etc).
- Aspirin lowers the risk of a heart attack.
- It is used for blood thinning.
Side-effects:
The overdose of aspirin shows different types of side effects like,
- Headache.
- Rash.
- Nausea.
- Bleeding.
- Ulceration.
- Heartburn.
- Stomach upset.
- Cramps.


Post a Comment